
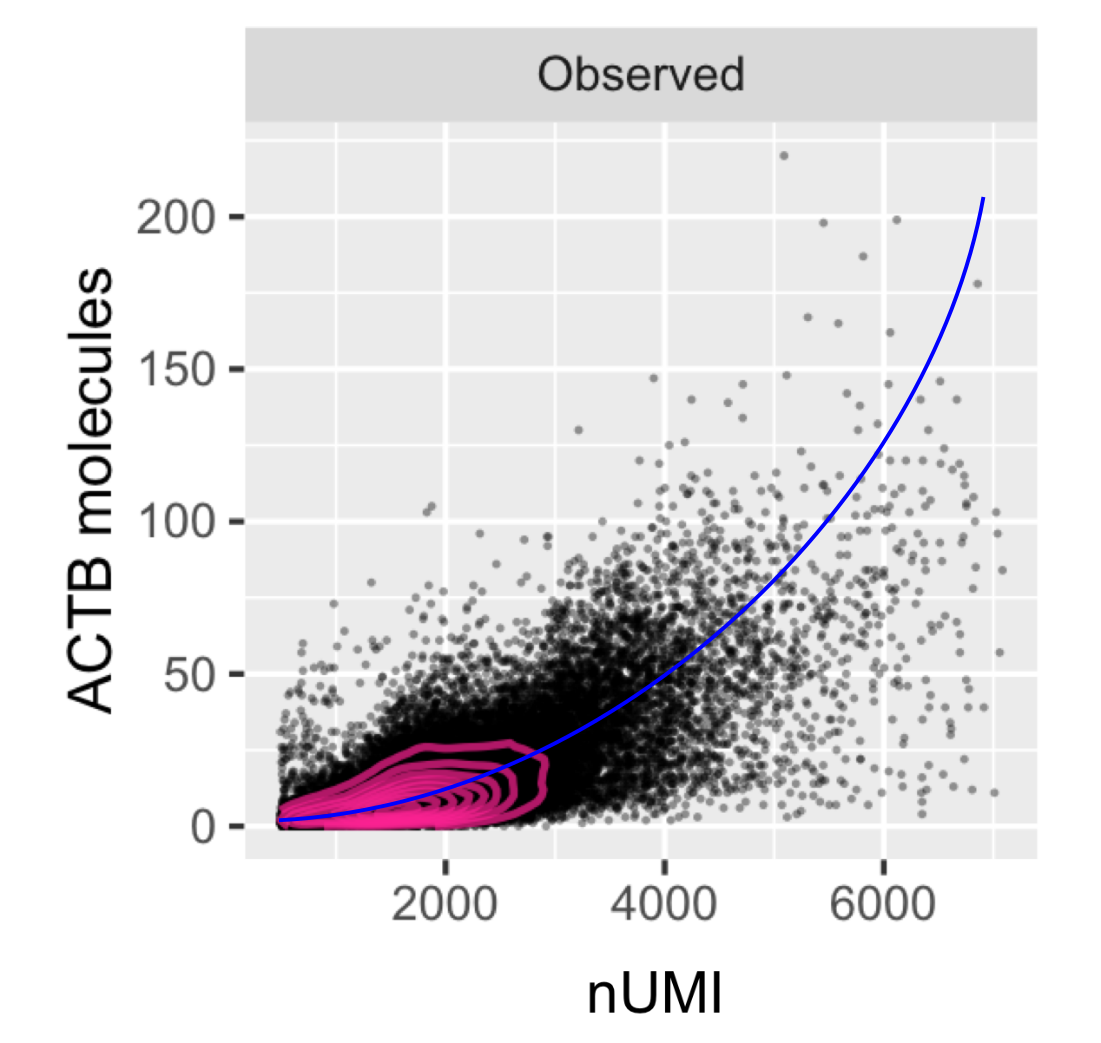
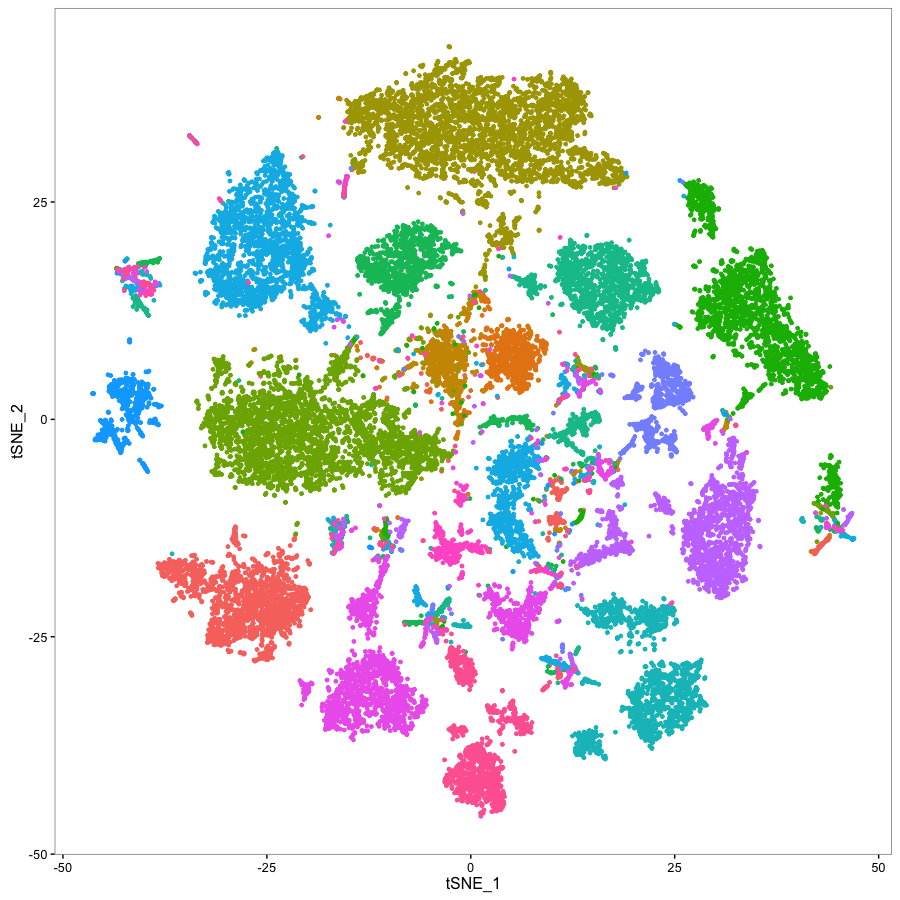
We study the causes and consequences of cellular heterogeneity in complex biological systems. Our group develops computational and experimental methods in single-cell and spatial genomics, integrating machine learning with multimodal measurements to understand how cells work together to drive biological processes, development, and disease.
Science moves fastest when tools, data, and ideas are shared openly. Through open-source software, freely shared technology, and livestreamed community events, we aim to empower researchers everywhere to explore single-cell biology and make discoveries of their own.
We direct an NIH Center for Excellence in Genomic Science (CEGS) to develop a comprehensive suite of computational and experimental tools for multimodal single cell analysis, and to share these with the broader community.
Single‑cell perturbation genomics
Single-cell perturbation screens are a new frontier for functional genomics—unlocking the ability to discover gene function, reconstruct regulatory networks, and build virtual cells. To realize this vision, we develop technologies for massively scalable, combinatorial, and multimodal perturbation profiling, alongside a new generation of computational tools to analyze, interpret, and integrate these rich datasets.
- CPA‑Perturb‑seq: systematic perturbation of cleavage and polyadenylation regulators (Kowalski et al., Cell 2024)
- Mixscape: performing and interpreting multimodal Perturb‑seq screens (Papalexi et al., Nat Genet 2021)
- Systematic and massively scalable perturbation profiling of signaling regulators (Jiang*, Dalgarno*, et al., Nat Cell Biol 2025)
- CaRPool‑seq: combinatorial targeting of RNA transcripts in single cells (Wessels et al., Nat Methods 2023)
Multimodal single‑cell genomics
RNA is only one facet of cell identity. A central challenge is to measure the many layers of molecular information—chromatin state, RNA expression, protein abundance, and more—from the same cell. By uniting these views, we can follow the flow of information across the central dogma, reveal mechanisms invisible to any single modality, and move closer to a truly complete portrait of cellular state.
We introduce technologies that jointly profile multiple molecular modalities across the central dogma.
- DOGMA‑Seq: tri‑modal ATAC+RNA+surface proteins (Mimitou et al., Nat Biotechnol 2021)
- NTT‑seq: multiplexed histone marks and surface proteins (Stuart et al., Nat Biotechnol 2022)
- scCUT&Tag‑pro: chromatin profiling with protein readouts (Zhang et al., Nat Biotechnol 2022)
- Phospho‑seq: multimodal profiling of intracellular/intranuclear proteins (Blair et al., Nat Commun 2025)
We develop computational methods to integrate multimodal data collected in the same cells or across diverse experiments
- Weighted Nearest Neighbor analysis for high‑resolution multimodal cell states (Hao et al., Cell 2021)
- Bridge Integration to align datasets across modalities (Hao et al., Nat Biotechnol 2023)
- Signac for single‑cell chromatin analysis (Stuart et al., Nat Methods 2021)
- Comprehensive integration of multimodal single‑cell and spatial data (Stuart*, Butler* et al., Cell 2019)
Constructing reference maps of the human body
Comprehensive reference maps are essential to understand the diversity of human cells in health and disease. We lead efforts within the NIH Human Biomolecular Atlas Project to build single-cell atlases across the human body. These maps provide a foundation for comparative analyses, enable the discovery of novel cell types and states, and serve as a reference framework for interpreting new datasets from any biological system.
- Pan-human Azimuth: Deep-learning enabled cell type annotation across tissues and technologies [software]
- Azimuth: Automated reference mapping of single-cell data [software]
- The future of automated single-cell data analysis (Lotfollahi*, Hao* et Cell 2024)
- Common Coordinate Frameworks for the human body (Rood et al Cell 2019)
Applications in immunity, development, and disease
We collaborate broadly to apply single‑cell approaches to immune regulation, vaccination and infection, hematopoiesis, neurodevelopment, autoimmunity, cancer, and hematological malignancies. Our goals are to reveal cellular states, circuits, and interactions that drive phenotypes in health and disease. We participate in multiple NIH Consortia, including the Cellular Senescence Network, and the Somatic Mosaicism across Human Tissues, and also collaborate closely with labs across the NYC area.
- SumRank: Identifying reproducible cell state changes in neurological disease (Nakatsuka*, et al., Nat Commun 2025)
- Multimodal characterization of antigen-specific T cells in COVID vaccination and disease (Zhang et al, Nat Immunol 2023)
- SMaHT: Somatic mosaicism across human tissues (SMaHT consortium, Nature 2025)
- Developmental diversification of inhibitory interneurons (Mayer*, Hafemeister*, Bandler* et al., Nature 2018)